INTERACTION BETWEEN WATER AND SALT(NaCl)
THIS VIDEO IS A COLLABORATION OF OUR TEAM, "BOSSKU BIOCHEMISTRY" WITH ANOTHER GROUP NAMED "RRQ" FROM LECTURE GROUP 2.
THIS VIDEO EXPLAIN ON HOW DOES INTERACTION OCCUR BETWEEN WATER AND SALT.
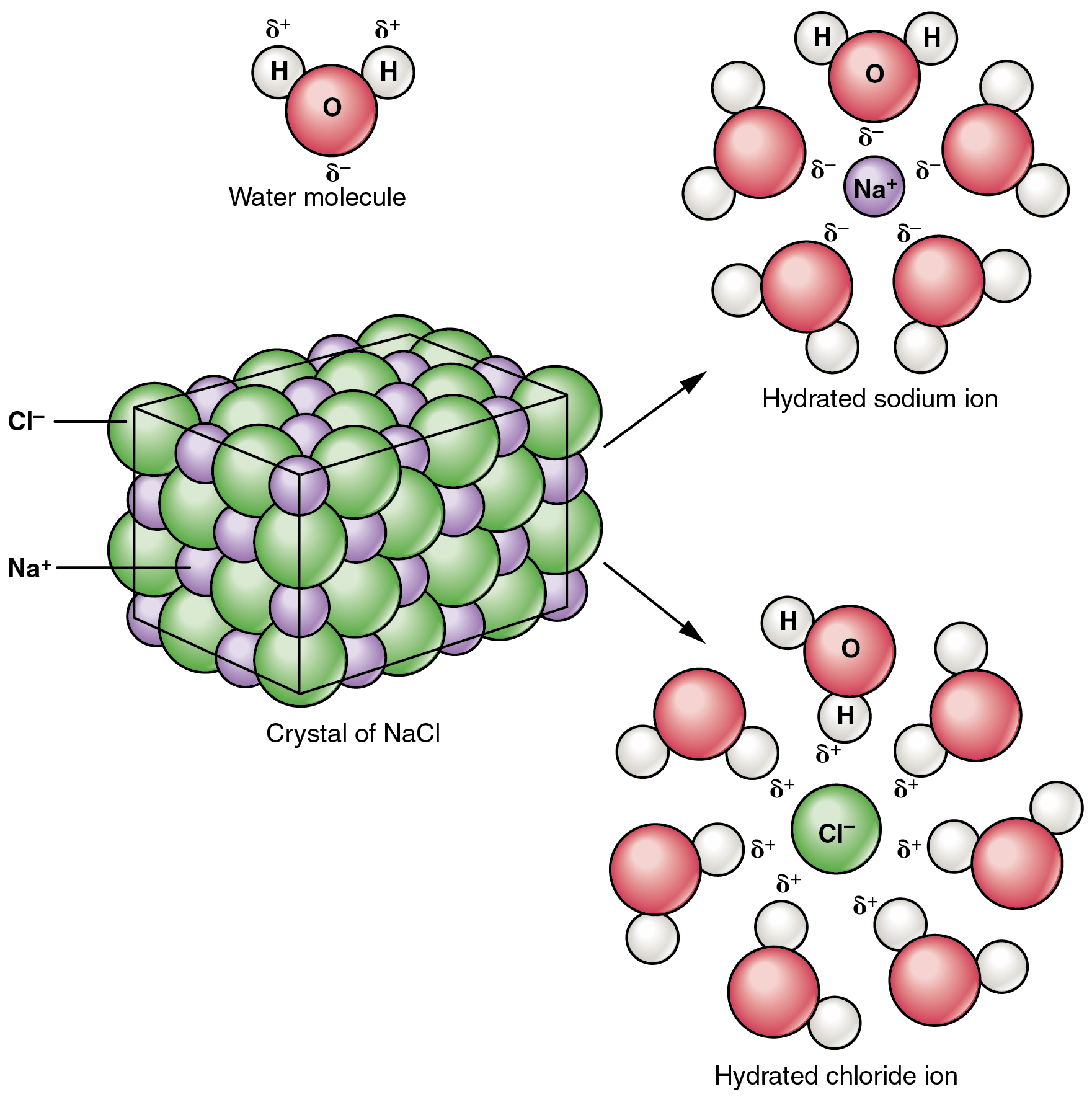
- NaCl is polar and in solid crystal form, Na is positively polar while Cl is negatively polar.
- Water, H2O is also a polar substance. H is positively charge while O is negatively charge.
- When NaCl added into water, Cl will attract H which positively charge and Na will attract O which negatively charge.
- NaCl will loss it crystal form. That's how crystal form dissolves in water.
RRQ X BOSSKU




Comments
Post a Comment